Abstract
Background: Real world data (RWD) is one of the key components to our understanding of the treatment trajectory of patients with multiple myeloma (MM) in clinical practice. However, treatment responses recorded in patients' medical records are not often standardized. We have developed an algorithm to derive real-world response (dR) for MM patients based on the International Myeloma Working Group (IMWG) criteria to overcome issues with missing values (Xu et al. Pharmacoepidemiol Drug Saf 2021). In this study, we evaluate its association with overall survival (OS) in a RWD cohort.
Methods: This study included patients diagnosed with MM between Jan 1, 2011 and Jan 31, 2021 from the US-based Flatiron Health electronic health record (EHR)-derived de-identified database. We derived overall response (partial response [PR] or better, e.g. > 50% reduction of serum M-protein from baseline) to 1L treatment in newly diagnosed MM patients based on the IMWG response criteria, taking into account missing data.
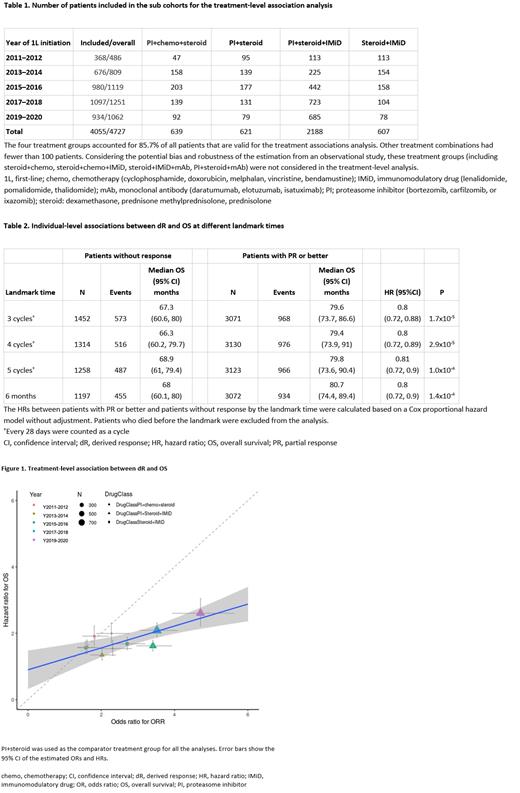
Associations between dR and OS were evaluated at individual- and treatment-level. At individual-level, associations were assessed using landmark analyses at approximating 3/4/5 cycles of treatment and at 6 months. Patients who died before the landmark were excluded from the analysis. At treatment-level, we stratified the cohort by year of treatment initiation (every two years from 2011-2021). Within each stratum, treatment groups were compared to estimate the hazard ratios (HRs) of OS and odds ratios (ORs) of dR, both adjusted for potential confounding factors (age, ECOG status, cytogenetic risk groups [high vs standard], time between diagnosis and first-line [1L] start date). The association between HRs and ORs was assessed by coefficient of determination (R 2) from a stratum-size weighted linear regression model, where values close to 1 imply a strong correlation and 0 indicates no association. Only significant HRs and ORs (P < 0.05) in the multivariable analysis were used in the analysis.
Results: Of 6806 patients in the Flatiron Health MM database, 70% (n=4830) had serum myeloma (M) protein measured by protein electrophoresis within 30 days before the start of 1L treatment, 27% (n=1838) had urine M protein and 57.8% (n=3935) had serum free light chains (FLCs). Patients with at least one serum M protein or FLC measurement were eligible for study entry (n=5609).
During 1L treatment, 4727 patients (46% female, mean ± standard deviation age 67.8 ± 10.4 years) had valid laboratory test results for dR assessment (for each patient we required a baseline measurement plus at least one additional measurement after treatment start of the same lab type), of which 71.7% (n=3387) had PR or better. The majority of the patients (n=2188, 46.3%) received a proteasome inhibitor (PI)+steroid+immunomodulatory drug (IMiD) treatment regimen, followed by PI+chemo+steroid (n=639, 13.5%), PI+steroid (n=621, 13.1%), and steroid+IMiD (n=607, 12.8%). Other treatment groups had fewer than 100 patients and were not considered in the treatment-level analysis (Table 1).
At the individual-level analysis, dR was significantly associated with OS at all landmarks (HRs 0.80 to 0.81, P < 0.001, Table 2). At treatment-level, the association between dR and OS was R 2=0.67 (P < 0.001, Figure 1). Subgroup analyses were conducted for each pair of treatment groups; however, only PI+IMiD+steroid vs PI+steroid had sufficient sample sizes, which showed R 2 of 0.82 (P = 0.02).
Conclusions: In the absence of recorded response data in a patient's EHR, laboratory assessments can successfully be applied using our algorithm to determine, based on part of the IMWG response criteria, the response status for patients with MM. In the RWD cohort, patients who had PR or better to 1L treatment demonstrated longer survival than non-responders. A moderate association was observed at the treatment-level between dR at 1L and OS. However, these associations might be specific to certain treatment groups and the underlying mode of action of the drugs. Further analysis will be needed to validate these results.
Xu: F. Hoffmann-La Roche AG: Current Employment. Tyanova: F. Hoffmann-La Roche: Current Employment. Williamson: Amgen: Current equity holder in publicly-traded company; Genentech: Current Employment, Current equity holder in publicly-traded company. Rocci: Novartis: Other: Wife is and employee of Novartis and holds Novartis stocks; Sanofi Aventis: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Received travel sponsorship ; AbbVie: Other: Received travel sponsorship ; F. Hoffmann-La Roche Ltd.: Current Employment; NHS: Ended employment in the past 24 months; Sanofi: Consultancy; Takeda: Consultancy, Honoraria, Other: Received travel sponsorship , Speakers Bureau; Roche: Current equity holder in publicly-traded company; Owner of 100% stocks of Harlock Healthcare Consulting Ltd (UK privately-held company currently not active).: Current holder of individual stocks in a privately-held company; Celgene: Honoraria, Other: Received travel sponsorship ; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag Ltd: Honoraria, Speakers Bureau. Yousefi: Roche: Current Employment, Current equity holder in publicly-traded company. Kumar: Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Antengene: Consultancy, Honoraria; Roche-Genentech: Consultancy, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Tenebio: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy; Carsgen: Research Funding; Beigene: Consultancy; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Bluebird Bio: Consultancy; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal